Recently, Yong Zhao's research group has made new progress in the field of lithium oxygen batteries, with the title of "greatly promoted oxygen reduction reaction activity of solid catalysts by regulating the stability of superoxide in metal-O2 batteries" published in the international academic Journal Science China Materials, 2020, Doi: s40843-020-1519-9, impact factor 6.098).
Paper links: https://engine.scichina.com/publisher/scp/journal/SCMs/doi/10.1007/s40843-020-1519-9?slug=abstract
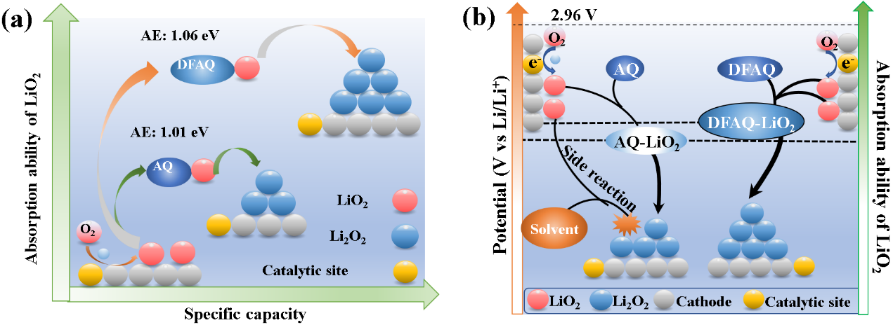
Aprotic lithium oxygen batteries have attracted worldwide attention due to their ultra-high theoretical energy density (3500 wh kg-1). In the process of oxygen reduction (ORR) on the positive side of the battery, oxygen is charged on the surface of the solid catalyst to form an adsorbed lithium peroxide (LiO2) intermediate. On the one hand, unstable LiO2 will form film-like and insulating lithium peroxide (Li2O2) through disproportionation or electroreduction reaction, resulting in the early termination of electrochemical oxygen reduction reaction; on the other hand, LiO2 will also react with solid catalysts (such as carbon materials) and electrolyte, thus passivating the electrode, resulting in discharge termination and poor cycle life. How to control the stability of LiO2 to ensure the continuous and efficient ORR on the surface of solid catalyst is a major challenge in the field of lithium oxygen battery (Chem. Soc. Rev., 2018, 47, 2921).
(a) the higher the adsorption energy of molecular additive-LiO2, the better the battery capacity; (b) the higher the adsorption energy of molecular additive-LiO2, the better the inhibition of side reaction induced by LiO2.
In the previous work, we put forward the research idea of ‘biomimetic enzyme coenzyme coordinated oxygen reduction catalytic reaction’. By adding anthraquinone molecules which can capture superoxide into the electrolyte, the formation of dense lithium peroxide film on the cathode surface was inhibited, and the oxygen reduction reaction on the surface of solid catalyst was promoted (J. Am. Chem. Soc., 2019, DOI:10.1021/jacs.8b13568). In this work, the stability of the discharge intermediate LiO2 was controlled by introducing different electron donating and withdrawing groups into the anthraquinone molecular framework. It was found that 1,4-difluoroanthaquinone (DFAQ) with electron withdrawing group had the highest adsorption strength for LiO2, which could stabilize LiO2 to the greatest extent, thus greatly improving the reaction rate and stability of surface ORR. The results show that the adsorption energy of anthraquinone cocatalyst for LiO2 has an important influence on the activity/ stability of ORR. This work not only provides a new technical means to regulate the stability of the intermediate product LiO2, but also helps to understand the mechanism of oxygen reduction in aprotic solvents (Sci. China Mater., 2020, DOI: s40843-020-1519-9).
Hua Wang and Dr. Liangliang Liu are the co-first authors of the paper, and Dr. Peng Zhang and Dr. Yong Zhao are the corresponding authors. This work is supported by the Organization Department of the CPC Central Committee, the National Natural Science Foundation of China, the Science and Technology Department of Henan Province, the Education Department of Henan Province and Henan University.